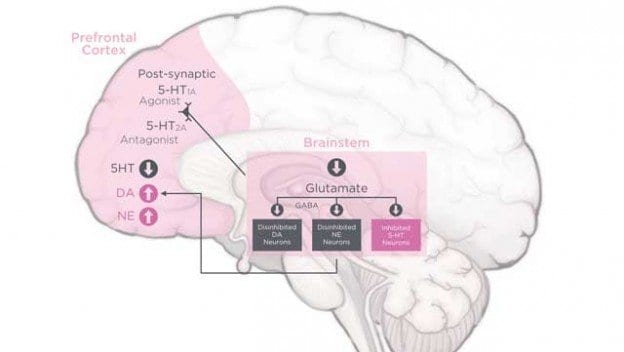
The Food and Drug Administration has received a new application from Sprout Pharmaceutical for approval of Flibanserin as a libido booster for women. The FDA has twice refused approval of Flibanserin because of side effects, which included fatigue, dizziness and nausea, and also because of lackluster effectiveness. Sprout re-filed its application and included new information that the FDA had requested from them about how Flibanserin affects the ability of women to drive.
Continue reading “Flibanserin Submitted For Another FDA Approval”