 Insulin glargine is an analog for human insulin, meaning that it is an altered form of insulin not produced by the human body, but still able to be used by the human body in a way similar to actual insulin. In the United States, insulin glargine is marketed by Sanofi-Aventus under the name Lantus. When used to supplement human insulin production, it is released in small concentrations over a 24 hour period into the blood, which means that it can be taken once a day for long-lasting insulin management
Insulin glargine is an analog for human insulin, meaning that it is an altered form of insulin not produced by the human body, but still able to be used by the human body in a way similar to actual insulin. In the United States, insulin glargine is marketed by Sanofi-Aventus under the name Lantus. When used to supplement human insulin production, it is released in small concentrations over a 24 hour period into the blood, which means that it can be taken once a day for long-lasting insulin management
Insulin glargine functions over time because it contains a modified domain that prolongs its interaction with the insulin-like growth factor-I receptor, or IGF-IR. However, many types of cancer patients show an overactivity of IGF-IR, leading some to believe that use of insulin glargine may be associated with the development cancer if it is continually interfacing with IGF-IR. But is there any truth to that hypothesis?
Researchers conducted 11 in vitro studies on human and mice cells to determine whether insulin glargine might cause the mitogenesis, or cell growth, of cancerous cells. The results showed that the insulin glargine promoted mitosis in 5 malignant cell cultures as well as one nonmalignant culture. Insulin glargine, overall, had a 10 percent to 60 percent greater mitogenic effect than regular human insulin.
Five more studies were conducted to investigate the risk that insulin glargine may have in causing cancer in humans. One study showed that treatment with insulin glargine alone was associated with a slightly higher risk of cancer in patients who had been diagnosed with Type 2 diabetes, with those receiving greater doses being more at-risk. However, a later analysis of the results that was adjusted for age and sex to ensure the objectivity of the results actually showed that insulin glargine had a protective effect on the study participants. The results were controversial and were heavily debated; however, three more studies were conducted which did not appear to show any increased risk of cancer from regular insulin glargine treatment.
The fifth and final study did find that female patients treated with insulin glargine alone appeared to show an increased risk for breast cancer compared to patients who had been treated with both insulin glargine and human insulin. Again, the findings of this study were the subject of debate as the authors did not take into consideration other risk factors for breast cancer, so the apparent increased risk of breast cancer may have simply been the result of random fluctuations in frequency. The authors came to the conclusion that the data did not provide a strong enough link to prove a causal relationship between insulin glargine use and the development of cancer compared to other types of insulin-based therapies.
The Food and Drug Administration released a new safety announcement in January 2011 regarding their review of insulin glargine and any possible links to cancer. The FDA determined that the evidence so far has been inconclusive since the studies and their methodology were questionable. However, the FDA continues to work with the medication‘s manufacturer as well as the Department of Veterans Affairs to determine whether there is a risk of developing cancer associated with the use of insulin glargine.
The American Diabetes Association, The American Association of Clinical Endocrinologists, the European Association for the Study of Diabetes, and the European Medicines Agency have all stated that there is currently no need for any change in the use of insulin glargine treatment.
 A study led by an Indian-origin researcher and published in the journal Nature
A study led by an Indian-origin researcher and published in the journal Nature 
 Scientists are currently performing tests on a new drug that may lead to the end of
Scientists are currently performing tests on a new drug that may lead to the end of 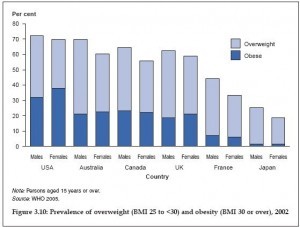 Obesity rates have been skyrocketing over the last three decades, and the epidemic shows no sign of slowing. While populations the world over have seen rising obesity rates, the West has been especially susceptible to the dangerously unhealthy rise in average weight, with all the health
Obesity rates have been skyrocketing over the last three decades, and the epidemic shows no sign of slowing. While populations the world over have seen rising obesity rates, the West has been especially susceptible to the dangerously unhealthy rise in average weight, with all the health  A meta-analysis of ten clinical trials has revealed that the consumption of dark chocolate and cocoa products, both of which are rich in polyphenol, may reduce total cholesterol and LDL cholesterol. However, consumption of these products has no effect on HDL cholesterol.
A meta-analysis of ten clinical trials has revealed that the consumption of dark chocolate and cocoa products, both of which are rich in polyphenol, may reduce total cholesterol and LDL cholesterol. However, consumption of these products has no effect on HDL cholesterol. U.S. consumers are more aware today that not all
U.S. consumers are more aware today that not all  The U.S. Institute of Medicine (IOM) has stated in a recent report that vaccines are overwhelmingly safe and that they do not cause diabetes or autism. The IOM came to the conclusion after reviewing over 1,000 published studies.
The U.S. Institute of Medicine (IOM) has stated in a recent report that vaccines are overwhelmingly safe and that they do not cause diabetes or autism. The IOM came to the conclusion after reviewing over 1,000 published studies. A high-tech diabetic safety service may soon use text messages to notify paramedics if a patient’s blood sugar levels dip too low. The program is being developed by researchers at Swansea University in Wales and scientists hope it reduce costs for the National Health Service in addition to saving the lives of diabetics.
A high-tech diabetic safety service may soon use text messages to notify paramedics if a patient’s blood sugar levels dip too low. The program is being developed by researchers at Swansea University in Wales and scientists hope it reduce costs for the National Health Service in addition to saving the lives of diabetics.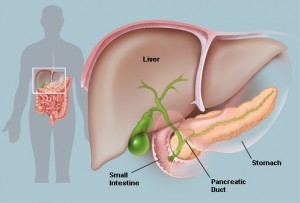 A research team lead by Professor Ulf Ahlgren and his colleagues is at the forefront of the development of optical projection tomography. Based in Umeå University in Sweden, the team has used this emerging technology to describe embryonic development of the pancreas and the distribution of the islets of Langerhans in the adult pancreas. The team’s findings will aid future research that utilizes modeling of human organs in treating diabetes.
A research team lead by Professor Ulf Ahlgren and his colleagues is at the forefront of the development of optical projection tomography. Based in Umeå University in Sweden, the team has used this emerging technology to describe embryonic development of the pancreas and the distribution of the islets of Langerhans in the adult pancreas. The team’s findings will aid future research that utilizes modeling of human organs in treating diabetes.