 A new kind of “ultrafast” insulin developed by Halozyme Therapeutics, Inc., has been shown in two clinical trials to work just as well as other insulin replacements in patients with both Type 1 and Type 2 diabetes.
A new kind of “ultrafast” insulin developed by Halozyme Therapeutics, Inc., has been shown in two clinical trials to work just as well as other insulin replacements in patients with both Type 1 and Type 2 diabetes.
According to Halozyme, the new insulin—called PH20—was as effective as Humalog, an Eli Lilly and Company product, at controlling blood glucose levels. Halozyme stated that PH20 was actually more effective at controlling postprandial glucose levels, or blood sugar levels after a meal. Hypoglycemia rates in PH20 users were similar to those of Humalog users. The two studies were Phase 2, mid-stage clinical trials, each using data from about 110 participants.
One study was conducted on patients with Type 1 diabetes while the other studied participants with Type 2 diabetes. The studies compared effects of PH20 with those of Humalog. PH20 is an insulin analog—a type of insulin that is not produced biologically but synthetically, yet functions the same in the human body as the insulin produced by the beta cells of the pancreas.
PH20 is derived from rHuPH20 (Lispro-PH20 or Aspart-PH20), a recombinant human hyaluronidase enzyme, which “temporarily degrades hyaluronan, a structural protein in the interstitial space,” according to Halozyme. The enzyme allows the delivery of therapeutic medications through subcutaneous injections rather than intravenously. According to Halozyme, subcutaneous injection of pharmaceuticals provides a variety of benefits for patients, including increased convenience and efficiency, extended product life cycle, and decreased costs.
The patients using PH20 insulin demonstrated more than a 50 percent increase in the number of patients who were able to consistently achieve post-mealtime blood glucose targets that are established by the American Association of Clinical Endocrinologists. Patients using PH20 insulin did not demonstrate a significant increase in hypoglycemia or hypoglycemic events; the events that did occur were typically mild and were similar to those experienced by patients using Humalog.
Halozyme stated that it will be pursuing methods of making PH20 available around the world, which means that the company may form a partnership with a larger pharmaceutical manufacturer. Halozyme will present the complete findings of the two studies at a medical conference in 2012. The news boosted Halozyme’s market presence, with shares 49 cents to $8.21 in afternoon trading, a 6.3 percent increase.
Halozyme’s shares also jumped recently by 18.6 percent when the company announced successful clinical trials of a breast cancer drug that Halozyme is developing with Swiss pharmaceutical company Roche. The drug is a variation of Herceptin, which is normally administered with an IV; Halozyme and Roche are working on an injectable version, which was shown in the trial to be just as effective as the IV version. The injectable version will offer more convenience to patients as it can be administered in about five minutes, while the IV version takes about 30 minutes.
According to Halozyme, the company is “dedicated to the development and commercialization of products targeting the area outside the cell known as the extracellular matrix,” a system that provides structural support for tissues and is responsible for directing some important biological activities in humans, such as cell migration, signaling, and survival. The importance of the matrix in biochemical processes allows Halozyme to target the system for treating illnesses. The company was founded in 1998 in San Diego and primarily targets the endocrinology, oncology, dermatology, and drug delivery markets.
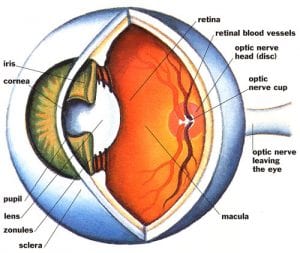 A recent study found that phacoemulsification, a process by which the internal lens of the eye is emulsified as a treatment for cataracts, does not affect intraocular pressure (IOP) in individuals who have diabetes. The findings were presented in a poster session at the American Academy of Opthalmology 2011 Annual Meeting, and they contradict previous research on the subject of phacoemulsification’s effects on intraocular pressure.
A recent study found that phacoemulsification, a process by which the internal lens of the eye is emulsified as a treatment for cataracts, does not affect intraocular pressure (IOP) in individuals who have diabetes. The findings were presented in a poster session at the American Academy of Opthalmology 2011 Annual Meeting, and they contradict previous research on the subject of phacoemulsification’s effects on intraocular pressure.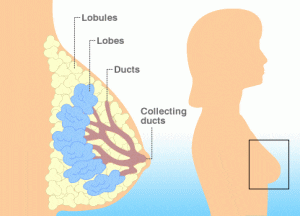 A new study conducted in Canada suggests that women who have been recently diagnosed with diabetes are more likely to also be diagnosed with breast cancer.
A new study conducted in Canada suggests that women who have been recently diagnosed with diabetes are more likely to also be diagnosed with breast cancer. The world will soon face a shortage of a quick-acting
The world will soon face a shortage of a quick-acting  Of all the factors that affect your health, your neighborhood may be the last thing you’d consider. But a recent study has discovered that the average economic status of a neighborhood’s residents are tied to their risk of obesity and diabetes. The researchers who discovered the association describe it as “modest but potentially important.”
Of all the factors that affect your health, your neighborhood may be the last thing you’d consider. But a recent study has discovered that the average economic status of a neighborhood’s residents are tied to their risk of obesity and diabetes. The researchers who discovered the association describe it as “modest but potentially important.” Amylin Pharamceuticals, Inc. recently won a significant victory as its Type 2 diabetes
Amylin Pharamceuticals, Inc. recently won a significant victory as its Type 2 diabetes  A biochemist at
A biochemist at  A $32.3 million rescue package has revitalized a revolutionary kind of Type 1 diabetes treatment that was on the ropes financially. The treatment uses transplanted pig cells to treat Type 1 diabetes and with the new cash infusion, the developers claim that the product will be on the market in New Zealand within three years’ time.
A $32.3 million rescue package has revitalized a revolutionary kind of Type 1 diabetes treatment that was on the ropes financially. The treatment uses transplanted pig cells to treat Type 1 diabetes and with the new cash infusion, the developers claim that the product will be on the market in New Zealand within three years’ time. Weight loss surgery appears to have benefits beyond reducing obesity—a key risk factor for Type 2 diabetes—in one individual. Surgery also promotes weight loss, healthy eating habits, and increased levels of physical activity among the family members of surgery recipients.
Weight loss surgery appears to have benefits beyond reducing obesity—a key risk factor for Type 2 diabetes—in one individual. Surgery also promotes weight loss, healthy eating habits, and increased levels of physical activity among the family members of surgery recipients. A recent Norwegian study found that lung cancer patients who also have diabetes often live longer than patients who do not have diabetes. The study was published in the “Journal of Thoracic Oncology,” the official journal associated with the International Association for the Study of Lung Cancer.
A recent Norwegian study found that lung cancer patients who also have diabetes often live longer than patients who do not have diabetes. The study was published in the “Journal of Thoracic Oncology,” the official journal associated with the International Association for the Study of Lung Cancer.