 The world will soon face a shortage of a quick-acting insulin product developed by Sanofi due to a manufacturing issue at a plant in Frankfurt.
The world will soon face a shortage of a quick-acting insulin product developed by Sanofi due to a manufacturing issue at a plant in Frankfurt.
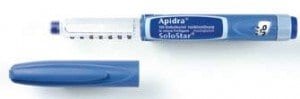
The product in question is the Apidra solostar pen, which is typically used alongside a meal by diabetics. The pen provides fast-acting insulin, which diabetics need so that they don’t experience dangerously high levels of blood glucose after eating a meal.
Sanofi wrote a letter to doctors about the shortage, and the letter was also posted on the Food and Drug Administration’s website. According to Sanofi, the manufacturing plant, located in Frankfurt, Germany, experienced a “technical incident” that affected production of Apidra pens. The incident occurred on July 11; Sanofi says that it is addressing the problem and expects that full production of Apidra pens will resume by the first quarter of 2012.
The letter noted that Apidra pens may become unavailable to U.S. residents later this month. Physicians can switch patients with diabetes to Apidra syringes and vials to replace the usage of the pens and will not have to make any adjustments to dosage.
Sanofi stated in its letter that it will offer free vials to patients with insurance who have co-pays through April. Information helpful to patients can be found on the official website of the Apidra pen, www.apidra.com.
Current users of Apidra pens need not worry, according to Sanofi: the manufacturing problem did not affect the pens that are currently available on the market. Lantus, a long-lasting insulin manufactured by Sanofi, is also not affected by the manufacturing issue.
Apidra is insulin glulisine, which is an insulin analogue—a type of synthetic insulin that does not occur naturally in any organisms but still performs all the functions of natural human insulin. The Apidra solostar pen delivers insulin glulisine through a subcutaneous injection; the insulin takes effect in humans faster than natural insulin does, and dosing is usually given in the 15 minutes before the individual eats a meal. Also known as a “mealtime” insulin, Apidra begins working about five minutes after the injection and continues to lower blood sugar levels for about two to four hours afterwards, as opposed to other types of insulin, which can be effective for up to 24 hours. The most common side effect of Apidra usage is hypoglycemia, or low blood sugar.
Sanofi may face increased competition in the United States due to the shortage of Apidra pens. Other fast-acting insulin products are available which could pick up the slack for the absence of Apidra pens. Patients with Type 1 diabetes require regular injections of insulin, often several per day, to ensure that blood glucose levels remain under control. Some Type 2 diabetics also require insulin injections.
Both variants of diabetes affect approximately 26 million Americans today. The disease is characterized by elevated blood glucose levels, which can cause tissue and nerve damage over time. Blood sugar levels remain elevated since the body either does not produce enough insulin to remove it from the bloodstream or it becomes resistant to the insulin that is produced. Type 1 diabetes occurs because the immune system attacks the beta cells of the pancreas, which are responsible for producing insulin. Type 1 diabetes is often diagnosed in children while Type 2 diabetes is more often diagnosed in adults and often associated with obesity, poor diet, lack of exercise and other unhealthy lifestyle choices.